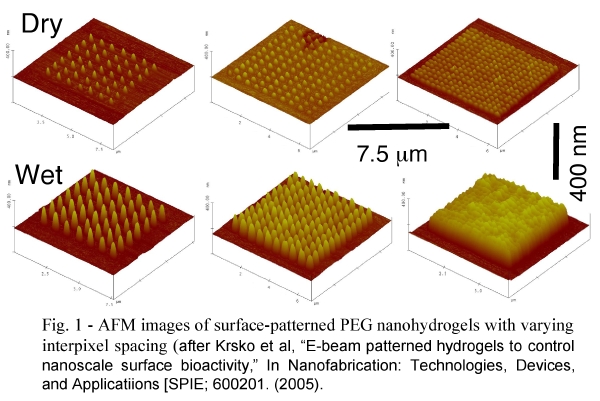
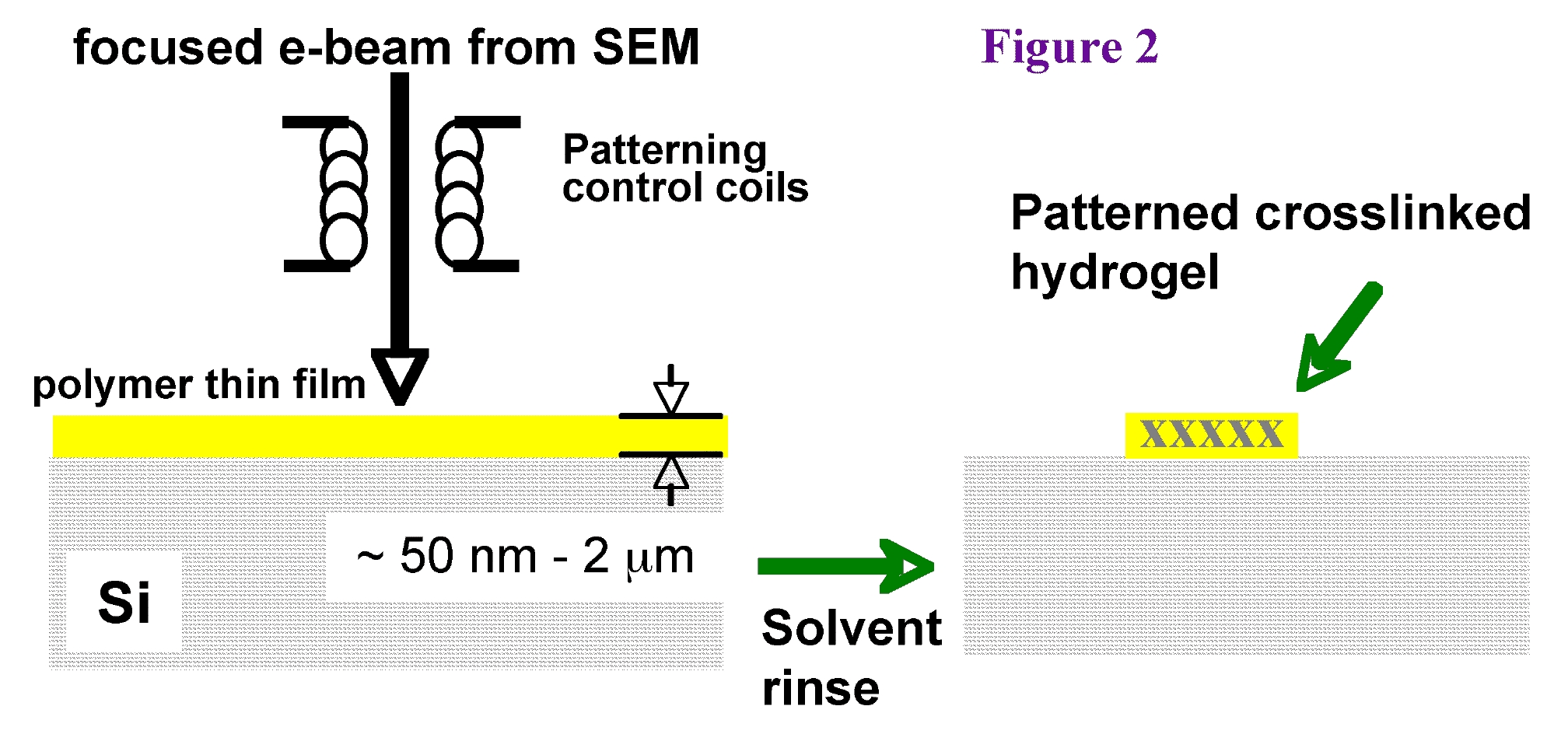
We have shown (Krsko et al., Langmuir 2003) that the swelling properties of the nanohydrogels can be controlled by the electron dose and the precursor molecular weight. We have also shown that high-swelling PEG nanohydrogels resist nonspecific adsorption. By incorporating functional groups (Hong et al, Langmuir 2004), such as amines, into the precursor we can create high-swelling nanohydrogels to which biomolecules such as proteins, antibodies, and oligonucleotides can be covalently grafted (figure 3).
Importantly, the flexible patterning capabilities of modern electron-optical systems not only enable us to make nanosclae feature sizes but also do so in user-defined patterns (fig. 3). Hence we can create surfaces with nanoscale biospecific features patterned at micro length scales. Such surfaces can be used in ultra-high-sensitivity detection applications as well as in controlling cell-surface interactions at cellular and sub-cellular length scales.
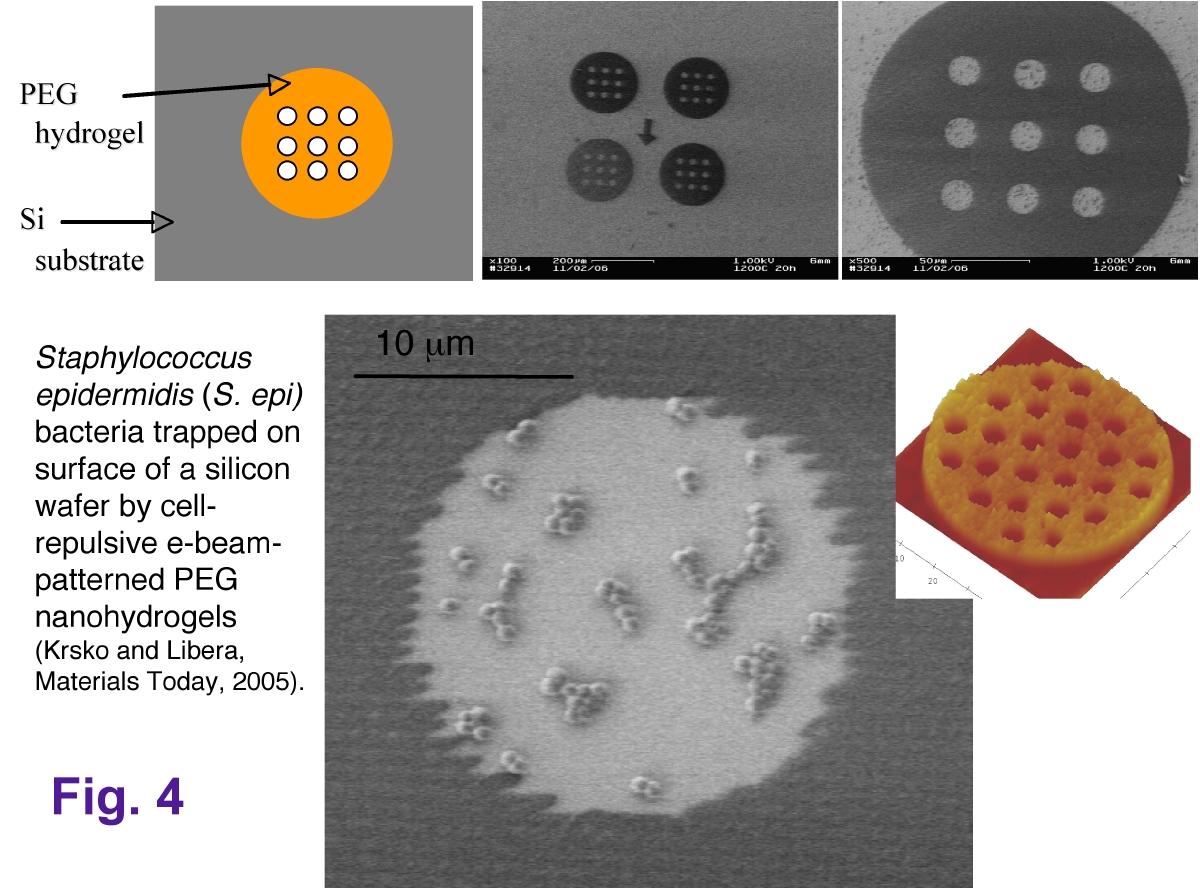
We are particularly interested in using surface-patterned nanohydrogels to control the interactions between synthetic surfaces, such as orthopaedic implant material, and bacteria (figure 4).
|
 |